Point of Care Devices Toolkit
The resources in this toolkit may only be used for internal improvement and education efforts. They may not be used for commercial purposes.
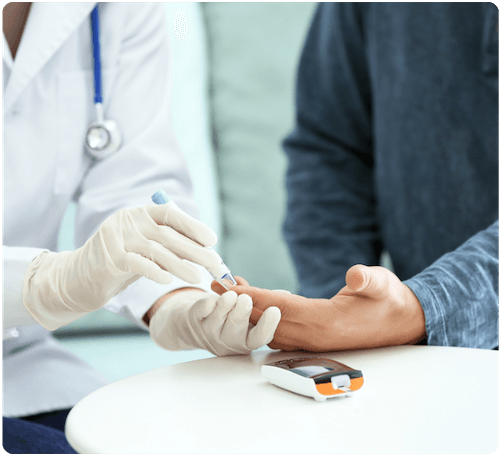
Point of care testing occurs at or near the site of patient care and is accomplished through the use of transportable, portable, and handheld instruments. Point of care testing devices, including blood glucose meters and lancing devices, must be used as directed in order to minimize the risk of transmitting blood-borne pathogens, including hepatitis B, hepatitis C, and HIV.
The ASC Quality Collaboration has assembled a variety of resources and information that may be used to supplement your current processes to improve infection prevention practices surrounding the use of point of care devices.
The Point of Care Devices Toolkit contains both essential resources and a broader array of materials, including:
Assessment Tools
- Point of Care Devices: What CMS Surveyors Are Looking For: Perform a self-assessment using the same tool CMS surveyors use when evaluating ASC use of point of care testing devices, such as glucometers.
- Injection Safety: Point of Care Testing (CDC): Though designed for use in a clinic, this assessment tool from the CDC can be used to observe and evaluate point of care testing practices in an ASC.
Implementation Aids
- Leadership Letter Template: This sample letter from clinical leaders in the ASC may be used to communicate plans for an awareness initiative regarding infection prevention in the use of point of care testing devices. The template should be adapted to fit the unique circumstances of each center.
- Policy and Procedure Template: Infection Prevention for Point of Care Testing: This sample infection prevention policy and procedure for point of care testing is based on the Centers for Disease Control and Prevention (CDC) guidelines. It should be adapted as needed for use in each facility.
- Summary of Glucometer Cleaning Guidelines (ASCP): Beginning on page 8, this 2010 document from the American Society of Consultant Pharmacists (ASCP) summarizes manufacturer cleaning guidelines for glucometers. Though prepared for the long term care environment, much of the information is applicable to the ASC setting.
- Registered Antimicrobial Products Effective Against Human HIV-1 and Hepatitis B Virus (EPA): This 2018 document from the Environmental Protection Agency (EPA) lists products that are registered as effective against HIV and Hepatitis B viruses.
Training Materials
- Infection Prevention in Point of Care Testing: This PowerPoint presentation touches on key infection prevention issues in the use of point of care testing and may be revised as desired to support the educational needs of the individual center.
- Preventing Infection During Blood Glucose Monitoring and Insulin Administration (OPSC): This 10-minute video from the Oregon Patient Safety Commission provides best practices that can be implemented in any healthcare setting during assisted blood glucose monitoring and insulin administration to prevent infection control breaches and the associated risk of spreading bloodborne pathogens.
Monitoring Tools
- Observation Template: Infection Control Practices during Point of Care Testing: This template may be used to observe infection control practices during point of care testing, such as blood glucose monitoring. It may be adapted as needed for use in the individual center.
Workplace Reminders
- Clean and Disinfect After Every Use Poster: This poster serves as a reminder to clean and disinfect point of care testing devices, such as glucose meters, after each use.
Guidelines from Leading Authorities
- Infection Prevention During Blood Glucose Monitoring and Insulin Administration (CDC): This webpage from the Centers for Disease Control and Prevention (CDC) provides essential guidance on infection prevention practices during blood glucose monitoring and insulin administration.
- Use of Fingerstick Devices on More than One Person Poses Risk for Transmitting Bloodborne Pathogens (CDC): This clinical reminder was developed by the Centers for Disease Control and Prevention (CDC) and reinforces guidance that fingerstick devices should never be used for more than one person.
- Guideline for Disinfection and Sterilization in Healthcare Facilities (CDC): This 2008 guideline was developed by the Centers for Disease Control and Prevention (CDC).
- Educating Providers and Persons with Diabetes to Prevent the Transmission of Bloodborne Infections and Avoid Injuries from Sharps (AADE): This position statement from the American Association of Diabetes Educators (AADE) includes infection control recommendations for professionals performing blood glucose fingerstick testing.
Resources Cited
The materials presented here include publicly available resources from the following organizations:
- Centers for Disease Control and Prevention (CDC)
- American Association of Diabetes Educators (AADE)
- Environmental Protection Agency (EPA)
- American Society of Consultant Pharmacists (ASCP)
- Oregon Patient Safety Commission (OPSC)
Comments and other feedback may be directed to Nina Goins, Executive Director, ASC Quality Collaboration.
Contact us today to learn more about how we can support you.
ASC Quality Collaboration
The only organization exclusively dedicated to advancing patient safety and quality of care delivery in ambulatory surgery centers.
